Contact Us
Tel: 0086 755 23248547
Fax: 0086 755 23248547
Skype:simon9969@hotmail.com
Linkman:Simon Gao
Postcode:518106
Web: www. injection-flow.com
E-mail:simon@mpa-tooling.com
Address: Building Yonghui, No. 202, Changchun north Road, Guangming new district, Shenzhen city, China
Medical
Solutions for Medical Device Manufacturers
Long development times and FDA (USA) , MHRA (UK) or TGA (AU) regulations often constrain the production design of a new medical device. MPA have conducted Mold flow analysis to identify the optimum feed points or solve molding problems without compromising regulatory approved design.
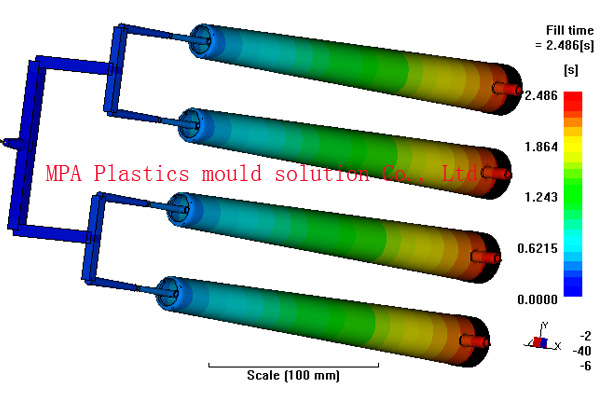
Asthma Inhaler
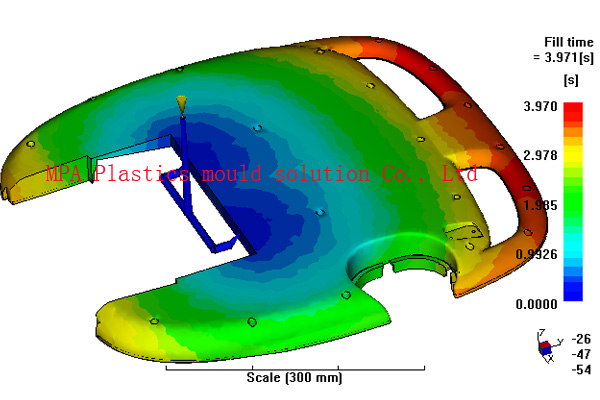
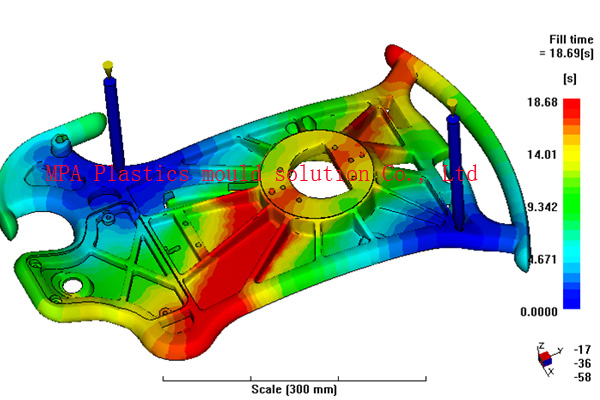
MPA have analyzed many different medical device components including inhalers, tablet and powder delivery devices, syringe parts, hearing aids, tracheotomy tubes and colostomy devices connection
Tablet Dispenser
Medical devices are often produced from exotic materials, fortunately the Mold flow includes extensive materials database and holds information on over 7500 different polymers and is expanding all the time. The database covers commodity materials to glass or carbon fiber filled engineered thermoplastics.
2005-2015 © Copyright MPA Mold flow analysis Tel:0086 755 23248547 Fax:0086 755 23248547
Sales Representative:simon@mpa-tooling.com